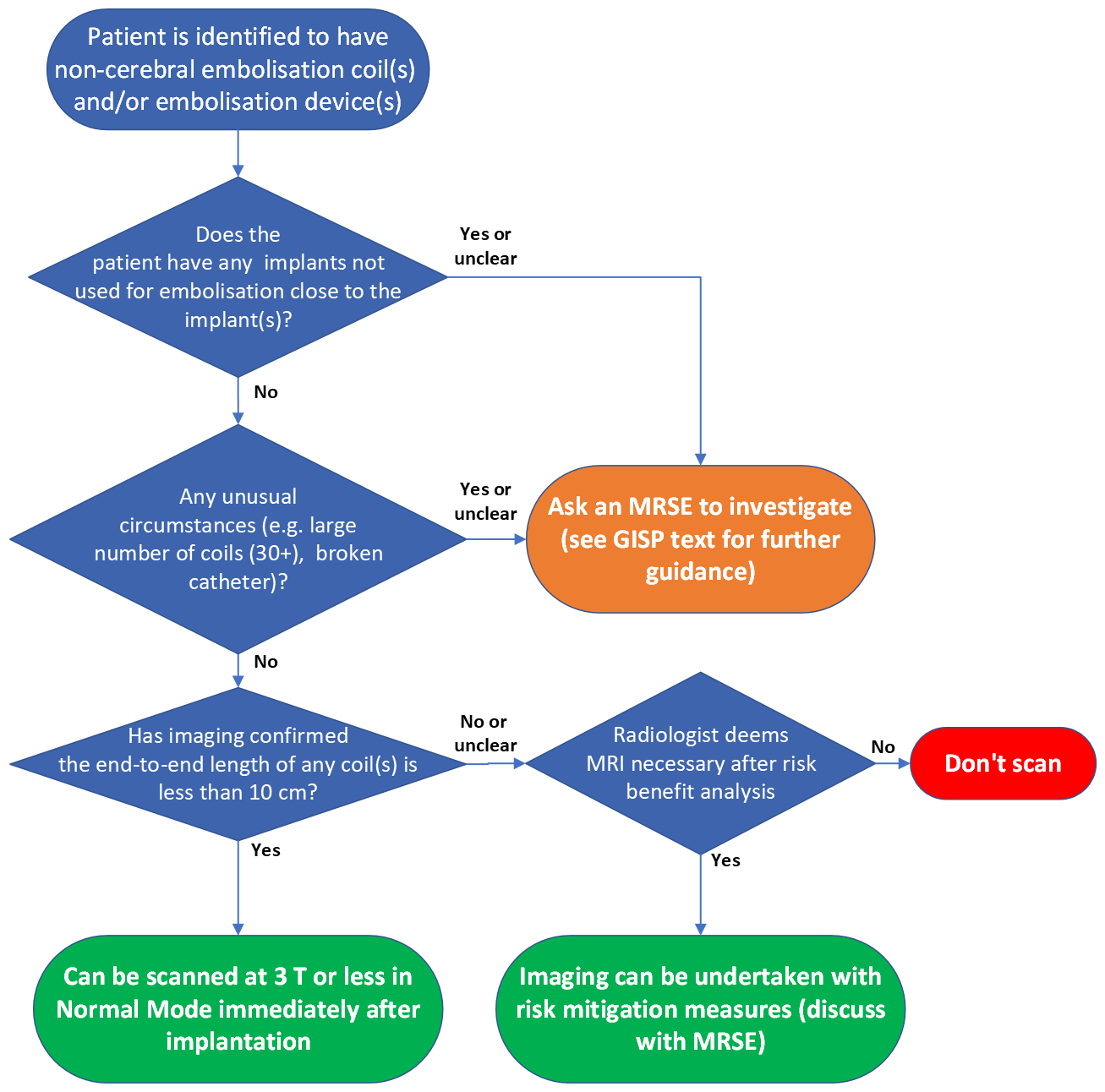
Information for patients
If you are a patient reading this and have a concern about an MRI scan you are scheduled to attend, we strongly recommend you contact the site where your scan is due to take place, you may also wish to refer to our ‘Information for Patients’ section. Please note local variations to the policies detailed on this website may apply, therefore please contact the hospital where your appointment is scheduled for clarification.Disclaimer (MUST READ)
The MRI safety information contained within this webpage is intended for use by staff from NHS Greater Glasgow and Clyde (GGC) and associated health boards, namely: NHS Ayrshire and Arran, NHS Borders, NHS Dumfries & Galloway, NHS Forth Valley, NHS Golden Jubilee, and NHS Lanarkshire. Only staff from these health boards are approved to use this information and local variations to the policies detailed may apply. Non-approved users i.e. patients and staff from health boards other than those listed above, or staff from private medical organisations use this information at their own risk. We, NHS GGC, accept no responsibility for patient injury or adverse outcomes that may occur as a consequence of the information contained herein. If you have any questions regarding this disclaimer, please contact the NHS GGC MRI physics team on: ggc.MRSafetyExpert@nhs.scot.NHS GGC MRI safety policy for scanning patients with non-cerebral vascular embolisation coils and embolisation devices
Brief summary of known devices
Non-cerebral vascular embolisation coils and embolisation devices.
Device use
Embolisation/aneurysm coils and devices are used to provide vascular occlusion, including the treatment for aneurysms. The device is inserted into the aneurysm or vessel, reducing blood flow and promoting clotting. Multiple coils are typically used for aneurysms and large vessel occlusion but have a range of uses including applications where the coil may be uncoiled or elongated.
Liquid embolic agents are rapidly solidifying agents that are also used to provide vascular occlusion, including into an aneurysm or AVM lumen.
Radioembolisation microspheres are glass or resin spheres containing Yttrium-90 that can be used to embolise unresectable tumours.
Must read: What this policy does not cover / notable exceptions
This GISP does not cover cerebral embolisation/aneurysm implants.
This GISP does not cover aneurysm clips, lung volume reduction coils nor situations where any implants other than stents are placed in close proximity to embolisation implants. Situations where the catheter is broken and retained are also excluded from this GISP and must be investigated on a case-by-case basis.
If a patient reports that their implant was inserted as part of a clinical trial or research study, these must be investigated on a case-by-case basis.
Embolisation coiling can be very inconsistent in the approaches used, if there are any unusual circumstances these should be considered outwith this GISP. These circumstances can be identified at the time of screening or from previous imaging and may include a large number of coils (30+) in close proximity. It is at the radiographer’s professional discretion what is deemed an “unusual circumstance”.
Must read: What the policy covers
This policy covers 1.5 and 3T MRI scanning of patients with non-cerebral vascular embolisation coils, liquid embolic devices and radioembolisation microspheres.
Must read: The MR safety policy
If available, prior imaging must be reviewed for all body embolisation to check whether a coil has been coiled and to ascertain the total length of all implants in close proximity.
If the implant(s) are confirmed to have an end-to-end length less than 10 cm (for 1.5 or 3T examinations), and the implants fall within the remit of the GISP as outlined above, the patient can undergo MR examination at 3T or less, in Normal Operating Mode, at any time after implantation. This advice is regardless of the value of the spatial gradient of the MRI scanner. Note that this information applies to cases in which the implants are in the area of the transmitted RF. If no clinical need to scan at 3T and 1.5T is available, scan at 1.5T.
If the end-to-end length of a coil or combination of coils exceeds 10 cm (for 1.5 or 3T examinations) or there is no prior imaging available then a risk-benefit analysis will be required. Guidance on the risk component is contained in the additional background information and discussion section of this policy. Risk mitigation approaches can be considered such as scanning on a lower field strength scanner, low SAR sequences, interleaving of low SAR and high SAR sequences or use of a transmit-receive RF coil (provided no part of the embolisation coil(s) are within the RF field). If no prior imaging, x-ray screening may be considered to determine the end-to-end length of the coil(s), particularly for patients with varicocele or ovarian coiling, to inform the risk component of the risk-benefit analysis.
For stent-assisted coiling, please also follow the non-coronary vascular stent policy.
Detailed review and risk assessments
The documentation underpinning this policy can be found here:
Embolisation coils and devices GISP documentation
An additional risk assessment related to but outwith the scope of this policy is included at the end of the GISP documentation. This additional risk assessment outlines the risk when the end-to-end length of a non-cerebral embolisation coils is longer than 10 cm.
Additional background information and discussion
The threshold of 10 cm is set because this is the longest length of any coil or bundle of coils reported during MRI safety testing (reported by Stryker). It is not well known what the risk of heating above these lengths are as no reports of MRI safety testing from manufacturers, the literature or any case studies or systematic reviews could be found. The theoretical risk of heating is present but given the lack of reported incidents, it should be deemed low risk such that if the referral is valid then the benefit will likely outweigh the risk.
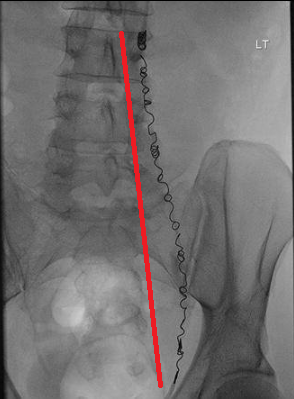
The end-to-end length of a coil can be measured on PACS. To do this, identify the image orientation that best displays the full length of the elongated coil and use the PACS measurement tool to draw a straight line from one end of the coil to the other, as demonstrated by the red line in the image below. If in any doubt, images should be reviewed by a Consultant Radiologist and further advice can be provided from MR Physics.
There will be significant susceptibility artefact from stainless steel embolisation coils that may limit the usefulness of the MR examination if the coils are near the area of interest. For platinum coils that are used more frequently, the susceptibility artefact is greatly reduced.