Information for patients
If you are a patient reading this and have a concern about an MRI scan you are scheduled to attend, we strongly recommend you contact the site where your scan is due to take place, you may also wish to refer to our ‘Information for Patients’ section. Please note local variations to the policies detailed on this website may apply, therefore please contact the hospital where your appointment is scheduled for clarification.Disclaimer (MUST READ)
The MRI safety information contained within this webpage is intended for use by staff from NHS Greater Glasgow and Clyde (GGC) and associated health boards, namely: NHS Ayrshire and Arran, NHS Borders, NHS Dumfries & Galloway, NHS Forth Valley, NHS Golden Jubilee, and NHS Lanarkshire. Only staff from these health boards are approved to use this information and local variations to the policies detailed may apply. Non-approved users i.e. patients and staff from health boards other than those listed above, or staff from private medical organisations use this information at their own risk. We, NHS GGC, accept no responsibility for patient injury or adverse outcomes that may occur as a consequence of the information contained herein. If you have any questions regarding this disclaimer, please contact the NHS GGC MRI physics team on: ggc.MRSafetyExpert@nhs.scot.NHS GGC MRI safety policy for patients with a Pulmonary Artery Band (PAB)
Must read: What this policy does not cover / notable exceptions
N/A
Must read: What the policy covers
This policy only applies to 1.5T and 3T MRI scanning of patients with a pulmonary artery band.
Must read: The MR safety policy
We are aware of at least one PAB that is MR Unsafe. Until a full review can be completed, we recommend the make and model of the PAB is identified and the MRI safety status of the specific PAB is established.
Conventional PABs use a non-metallic band and are MR Safe. If the make and model of the PAB cannot be identified, please ask a Radiologist to review previous imaging to determine if there is any metal present. Please see the additional information for more on the MR Unsafe PAB and its appearance on x-ray.
Risk assessment: A risk assessment underpinning this policy can be found here: GenericRiskAssessmentForm_pulmonary_artery_bands
Additional background information and discussion
Pulmonary artery banding (PAB) is a palliative procedure that is used as part of staged treatment before definitive surgical correction of congenital heart defects. This is typically performed on infants to allow surgical correction to take place when the infant is big enough. The conventional technique of PAB involves surgical placement of a (not telemetrically adjustable) band around the main pulmonary artery. Different techniques using a variety of materials (such as strips of polytetrafluoroethylene, polydioxanone or nylon) and sutures are used. In non-adjustable PAB methods, reoperation is often needed to adjust the tightness of the band. Given the advances in surgical techniques, PAB is only used for complex congenital heart defects nowadays.
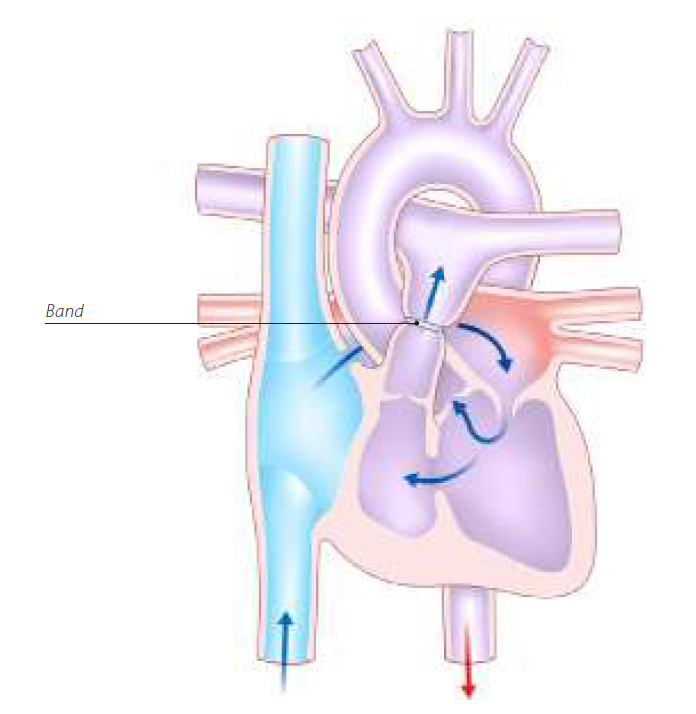
The overwhelming majority of pulmonary artery bands are MR Safe or MR Conditional with conditions that are fairly typical and straightforward to satisfy. However, there exists a telemetrically adjustable PAB that is MR Unsafe. The known device of concern is the AbbVie/Allergan/Endoart Medical Technologies, FloWatch PAB device.
FloWatch telemetric control systems use RF to adjust medical implants such as their pulmonary artery band (used in paediatrics) or their Easyband gastric band. It is unclear whether the FloWatch PAB went beyond clinical trials but some articles suggested it was being considered for routine use for particular paediatric patients.
AbbVie (who bought Allergan/Endoart) were asked to provide further information on the FloWatch PAB. Their response was: “This product is not marketed/licensed in the United Kingdom (UK), therefore it is not available in the U.K.” and were unwilling or unable to comment further.
From the information we could gather, the FloWatch was installed in a series of multi-national clinical trials including Europe and Asia. In the UK, the majority of FloWatch PAB devices were implanted in Alder Hey in Liverpool. An estimated 300 devices were implanted across 12 different countries. We understand that these devices were implanted between approximately between 2001 and 2013 but it could be later than this as the NICE guidance related to this telemetric PAB technique mentions that the FloWatch device was no longer available in January 2018 (https://www.nice.org.uk/guidance/ipg505). They also mention in their guidance that “the band is removed at the same time as cardiac surgery for definitive repair of any heart defects”. Furthermore, the conditions needing pulmonary artery banding are rare. Unfortunately, it is not known how many of these devices are still implanted but a clinician who was involved in some of the clinical trials estimated that there would be very few patients with this device still in situ.
The images below are taken from Kalavrouziotis et al (2008), which documented the first application of the FloWatch PAB device in Greece. These images may be useful for x-ray identification of the device.

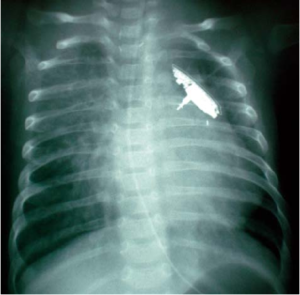
It is important to note that a detailed review of all PABs has not yet been performed and therefore there may be other MR Unsafe PAB devices implanted.