Summary
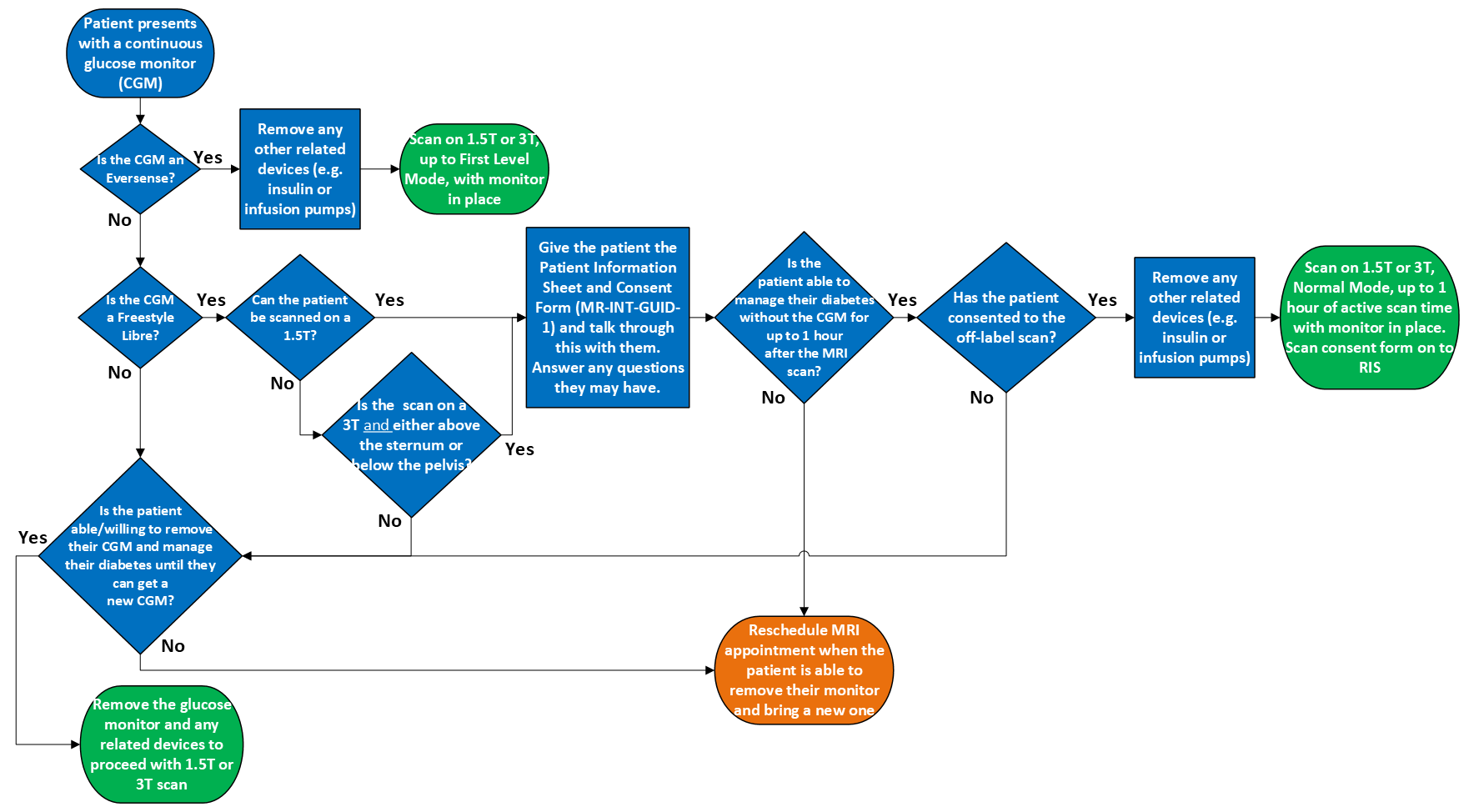
There is currently one CGM sensor that is MR Conditional in the UK, the Eversense (and Eversense XL) CGM sensor. Please see further details below.
In addition, an off-label risk assessment and Patient Information Sheet and Consent Form has been written to allow the Abbott Freestyle Libre CGM sensors to remain during MRI provided the patient provides informed consent. Please see further details below.
The remaining glucose monitors are currently classed as MR Unsafe and must be removed before the scan can proceed (for a general MRI risk assessment for glucose monitors, see DR-GGC-RISK-077.
Decision support workflow for determining whether or not to perform MRI on patients with a continuous glucose monitor
Abbott Freestyle Libre CGMs
Abbott Freestyle Libre CGM sensors (The Libre 2, Libre 2 Plus, Libre 3, and Libre 3 Plus) are MR Conditional in the United States and are identical to the CGM sensors of the same name in the UK. Given the risk of keeping this particular CGM has been demonstrated to be low, we have developed an off-label process for scanning patients with these CGMs. Please see the following risk assessment (DR-GGC-RISK-214) and Patient Information Sheet and Consent Form (MR-INT-GUID-1) for more details.
Detailed US MRI Scanning Conditions can be found at https://www.freestyle.abbott/us-en/safety-information.html, with a summary below:
- Magnetic Field Strength: 1.5T and 3T
- Operating Mode: Normal Operating Mode
- RF Excitation: Circularly Polarised (CP)
- RF Transmit Coil Type: Integrated Whole Body Transmit Coil
- Scan Duration
- 1.5T, all locations
- Up to 1 hour of continuous scanning without cooling period.
- 3T, between the pelvis and the sternum
- Up to 12 minutes of scanning with a cooling period of 2 minutes between scans.
- 3T, above sternum and below pelvis
- Up to 1 hour of continuous scanning without cooling period.
- 1.5T, all locations
Please note that there is an expected artefact of up to 5.8cm or 6.9cm, depending on the Libre Sensor model.
Eversense CGM Sensor
The Eversense (and Eversense XL) CGM sensor is MR Conditional in the UK. Despite being an MR Conditional device, please note that the smart transmitter component for this device is MR Unsafe and must be removed. Please see the notes below for more information on the sensor and transmitter. The conditions for the sensor are as follows:
• The Eversense Smart Transmitter must be removed before undergoing an MRI procedure
• Static magnetic field of 1.5 or 3.0T
• Maximum spatial field gradient of 19 T/m (1900 Gauss/cm)
• Maximum MR system reported, whole body averaged specific absorption rate (SAR) of 4 W/kg (First Level Controlled Operating Mode) for 15 minutes of continuous scanning, or SAR of 2 W/kg (equivalent to Normal Operating Mode) for 30 minutes of continuous scanning.
This advice will be reviewed on a regular basis. Should you have any queries or information to update this policy, please email MRI Physics.
Additional background information and discussion
The Eversense and Eversense XL Sensors are the component that is inserted under the skin (upper arm) and last up to 90 and 180 days, respectively. The Smart Transmitter is removable and worn externally over the sensor. This powers the sensor and sends data via Bluetooth to a mobile device. The Smart Transmitter is MR Unsafe and must be removed prior to entry into the MR Environment.
