Information for patients
If you are a patient reading this and have a concern about an MRI scan you are scheduled to attend, we strongly recommend you contact the site where your scan is due to take place, you may also wish to refer to our ‘Information for Patients’ section. Please note local variations to the policies detailed on this website may apply, therefore please contact the hospital where your appointment is scheduled for clarification.
Disclaimer (MUST READ)
The MRI safety information contained within this webpage is intended for use by staff from NHS Greater Glasgow and Clyde (GGC) and associated health boards, namely: NHS Ayrshire and Arran, NHS Borders, NHS Dumfries & Galloway, NHS Forth Valley, NHS Golden Jubilee, and NHS Lanarkshire. Only staff from these health boards are approved to use this information and local variations to the policies detailed may apply. Non-approved users i.e. patients and staff from health boards other than those listed above, or staff from private medical organisations use this information at their own risk. We, NHS GGC, accept no responsibility for patient injury or adverse outcomes that may occur as a consequence of the information contained herein. If you have any questions regarding this disclaimer, please contact the NHS GGC MRI physics team on: ggc.MRSafetyExpert@nhs.scot.
MRI safety policy for patients with CSF shunts e.g. for hydrocephalus
Brief summary of known devices
- The majority of known non-programmable CSF shunt valves are MR Safe or MR Conditional up to 3T.
- The above is based on the evidence that most devices are non-metallic in design (e.g. silicone, polypropylene) or are comprised of non-ferromagnetic materials (e.g. titanium, tantalum, 316L stainless steel) and contain no electrically conductive nor magnetic active components.
Must read: What this policy does not cover / notable exceptions
- This policy does not cover non-CSF shunts e.g. shunts used for Trans-jugular intrahepatic portosystemic shunt (TIPSS) (often in the Liver) i.e. vascular shunts or shunts to treat ascites e.g. to treat excess fluid in the abdomen.
- Programmable CSF shunts – they require make and model identification for the MR Conditions to be determined.
- External accessories e.g., programming tools, tools used to make pressure measurements/evaluation of CSF which are considered MR Unsafe and therefore prohibited from entering the MR Environment.
Must read: What the policy covers
- Non-programmable CSF shunt valve and catheter system, where the specific make and model of the implant may not be known.
- All non-programmable CSF shunt valve systems irrespective of year of implantation or implantation centre location.
- 1.5T and 3T MRI scanning of patient with non-programmable CSF shunt valves
Must read: The MR safety policy
- A non-programmable CSF shunt valve and catheter system can be scanned immediately after implantation at 1.5T or 3T in normal operating mode.
Risk assessment: A risk assessment underpinning this policy can be found here:
GenericRiskAssessmentForm_non_programmable_shunts
Link to detailed review
Additional background information and discussion
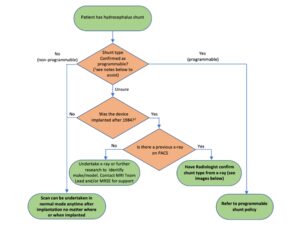
Care must be taken to confirm a patient’s shunt valve is indeed non-programmable, and that this is not confused with programmable shunt valves which incorporate a magnetic active component in their design.
The reader is directed to the flow chart below to help navigate the decision-making process in determining and confirming whether a patient’s shunt system involves a non-programmable valve.
1This can be determined from the referrer’s request, patient notes or from the patient implant card. If the patient claims to know their shunt type, then you must be assured that this is correct. The following information may help:
- For programmable shunts, the patient should be aware of a neurosurgeon/doctor using a magnetic programming device over the implanted region (most commonly the head) to change/check the valve settings. If implanted recently (i.e. within approximately 6 weeks), this may not have been done.
- For programmable shunts, the patient should be warned about their shunt being affected by external magnets (e.g. headphones, mobile phones etc). This does not apply to airport scanners since patients with a programmable or non-programmable shun will be warned of these.
- If the patent’s shunt is described as a fixed pressure or as a “low”, “medium”, or “high” pressure valve, it is likely a non-programmable valve. If described as “adjustable”, the shunt valve is programmable.
- If previous MR images have been performed over the implant region, i.e. head, these may be assessed for significant metal-induced artefact to determine whether the patient has a programmable shunt valve. Since programmable shunt valves include a magnetic component, significant signal void would present compared with a non-programmable shunt valve.
2Programmable shunt valves were not implanted prior to this date.
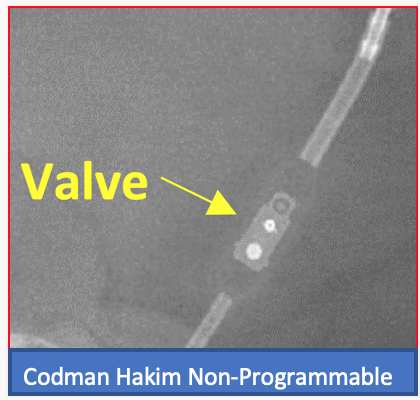
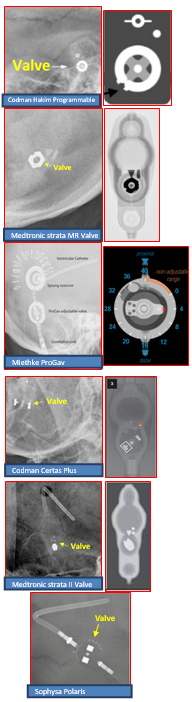
Programmable shunts can be identified on X-ray imaging via the cam assembly component which typically has a notch or indicator highlighting the shunt setting. This is not present on non-programmable shunt valves. Examples images are shown below. We would also recommend the AJNR paper by Lollis et al on programmable shunt radiographic identification: https://www.ajnr.org/content/31/7/1343.
Acknowledgement
This policy was created by members of the MRI subgroup of the Medical Physics and Clinical Engineering (MPCE: MRI) network for NHS Scotland in collaboration with the Scottish MR radiographer leads group.