Information for patients
If you are a patient reading this and have a concern about an MRI scan you are scheduled to attend, we strongly recommend you contact the site where your scan is due to take place, you may also wish to refer to our ‘Information for Patients’ section. Please note local variations to the policies detailed on this website may apply, therefore please contact the hospital where your appointment is scheduled for clarification.
Disclaimer (MUST READ)
The MRI safety information contained within this webpage is intended for use by staff from NHS Greater Glasgow and Clyde (GGC) and associated health boards, namely: NHS Ayrshire and Arran, NHS Borders, NHS Dumfries & Galloway, NHS Forth Valley, NHS Golden Jubilee, and NHS Lanarkshire. Only staff from these health boards are approved to use this information and local variations to the policies detailed may apply. Non-approved users i.e. patients and staff from health boards other than those listed above, or staff from private medical organisations use this information at their own risk. We, NHS GGC, accept no responsibility for patient injury or adverse outcomes that may occur as a consequence of the information contained herein. If you have any questions regarding this disclaimer, please contact the NHS GGC MRI physics team on: ggc.MRSafetyExpert@nhs.scot.
Patients with intraorbital eye lens (IOL) implants
Device Use
- Artificial eye lens implants or “intraocular lens” (IOL) are implanted following cataract removal or during refractive lens surgery.
- Cataracts causes the natural lens of the eye to become cloudy, resulting in vision deterioration. The natural eye lens is removed, and an artificial lens inserted in it’s place. In the case of refractive lens surgery, the procedure is similar to cataracts surgery where a refractive eye lens is implanted to correct short or long-sightedness.
- The basic components of an IOL implant include the central optic portion and two haptics that hold the device in position. This prevents the lens from dislocation which eventually becomes encased and secured by fibrous tissue.
Must read: What this policy does not cover / notable exceptions
- Other orbital eye implants not inserted for reasons of cataract surgery or refractive lens surgery. This includes, but is not limited to retinal tacks, scleral buckles, eyelid weights/springs and Glaucoma devices.
- Contacts lenses.
Must read: What the policy covers
- Artificial eye lens implants inserted during cataract surgery and/or refractive lens surgery.
- All artificial eye lens implants irrespective of year of implantation or implantation centre location.
Must read: The MR safety policy
An artificial eye lens implants can be scanned at 1.5T or 3T in normal mode.
Risk assessment: A risk assessment underpinning this policy can be found here
Detailed review: A detailed review underpinning this policy can be found here
Additional background information and discussion
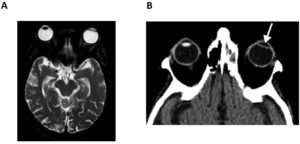
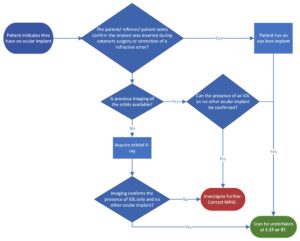
Care must be taken to confirm a patient’s orbital implant is indeed an artificial eye lens implant, and that this is not confused with other orbital implants for which a wide range exists and some which are labelled MR Unsafe.
The reader is directed to the flow chart (Fig. 1), and the CT/MR images (Fig. 2) below to help navigate the decision-making process in determining and confirming whether a patient has an artificial eye lens implant.