Information for patients
If you are a patient reading this and have a concern about an MRI scan you are scheduled to attend, we strongly recommend you contact the site where your scan is due to take place, you may also wish to refer to our ‘Information for Patients’ section. Please note local variations to the policies detailed on this website may apply, therefore please contact the hospital where your appointment is scheduled for clarification.
Disclaimer (MUST READ)
The MRI safety information contained within this webpage is intended for use by staff from NHS Greater Glasgow and Clyde (GGC) and associated health boards, namely: NHS Ayrshire and Arran, NHS Borders, NHS Dumfries & Galloway, NHS Forth Valley, NHS Golden Jubilee, and NHS Lanarkshire. Only staff from these health boards are approved to use this information and local variations to the policies detailed may apply. Non-approved users i.e. patients and staff from health boards other than those listed above, or staff from private medical organisations use this information at their own risk. We, NHS GGC, accept no responsibility for patient injury or adverse outcomes that may occur as a consequence of the information contained herein. If you have any questions regarding this disclaimer, please contact the NHS GGC MRI physics team on: ggc.MRSafetyExpert@nhs.scot.
NHS GGC MRI Safety policy for scanning patients with Fixed Internal, passive orthopaedic implants
Brief Summary
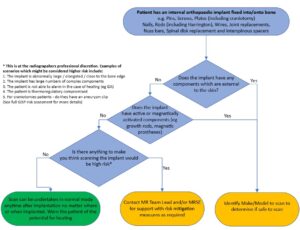
Device Use
Orthopaedic implants help treat and support damaged bones and other orthopaedic structures. These implants can be solely internal or have both internal and external elements (although those with external elements typically are only for temporary use). They vary vastly in size and shape and include pins plates, joint replacements, sternal closure devices, spine correction devices (e.g Harrington rods scoliosis), rib correction devices (e.g Nuss bar for pectus excavatum) to name a few.
Due to the highly variable shape and size of bone structures in the human body, these implants are manufactured in a wide variety of configurations. Many implants are provided as variable modular components that can be constructed to satisfy the requirements of individual patients. As such MR labelling is often not provided for many orthopaedic devices.
Modern metallic versions of orthopaedic implants are typically made from Titanium, Cobalt Chromium or austenitic stainless steel.
Must read: What this policy does not cover / notable exceptions
- Active devices such as magnetically activated implants (e.g MAGEC device).
- Any orthopaedic implant with external components (e.g external fixation device).
- Implants which are not well secured into bone.
- Implants that are extremely and abnormally large or have particularly complex configuration (deciding on this is as per radiographers professional discretion).
Must read: What the policy covers
All internal orthopaedic implants which are fixed or constrained into/onto bone. This includes (but not limited to):
- Pins, Screws, Plates (including craniotomy)
- Nails and Rods (including Harrington)
- Wires (including sternal)
- Sternal fixation devices
- Joint replacements
- Nuss bars
- Spinal disk replacement and interspinous spacers
Must read: The MR safety policy
- Patients are given the squeeze alarm such that they could inform the radiographer should they feel discomfort.
- Patient must be scanned in normal mode.
- Patients are screened to ensure to accurate information regarding the nature of the device avoiding the confusion between passive and active devices. If there is any uncertainty the device should be further investigated.
- Prevention of contact with scanner bore by the use of non-conducting foam pads of 2cm thickness.
- Prevention of induction loops by careful positioning of the patient and use of non-conducting foam pads at potential contact points between tissue e.g. thigh-thigh or between tissue and equipment cables.
- Restricting the scan duration to the minimum required for diagnosis.
- Interleaving high SAR scans with low SAR scans or generally leaving a time gap between applied sequences.
- Where possible and for higher risk cases use of a Transmit receive coil to image an area away from the implant site.
Additional focus on reducing SAR to mitigate heating should be considered in the following higher risk cases:
- Individuals with a compromised thermoregulatory response
- Individuals who are unable to provide immediate feedback on heating sensation such as patients under general anaesthetic or sedation or with disabled or partially dysfunctional perfusion abilities or dysfunctional heat sensation.
- Individuals who are anatomically large, broad, or obese They will likely be closer to the scanner bore (and hence to the body RF transmit coil) and may also provide a larger induced current path.
- MR imaging when the landmark position is over the implant.
- Devices which seem abnormally large or have multiple complex components particularly when these components have short gaps between them.
- When an elongated implant is positioned parallel to the distribution of the induced electric field in the body and in regions of high electric field. Electric field distribution will vary with body tissue distribution. Generally, for a landmark position at lower thorax/upper abdomen, an elongated implant at the periphery of the body or across shoulders or pelvis may meet this condition
A detailed review can be found here
The risk assessment for patients with these implants who are to be scanned under normal circumstances can be found here:
GenericRiskAssessmentForm_fixed_ortho_implants
The risk assessment for patients with these implants who are under sedation or GA can be found here:
GenericRiskAssessmentForm_fixed_ortho_implants_under_GA
Acknowledgement
This policy was created by members of the MRI subgroup of the Medical Physics and Clinical Engineering (MPCE) network for NHS Scotland in collaboration with the Scottish MR radiographer leads group.